LBussy
Well-Known Member
That is the Lotioncrafter MSDS. I used the supplier's MSDS: http://gmzcare.com/wp-content/uploads/2014/09/TD_emersol_7036.pdf (which I also linked) I don't know why there's a difference but Jen told me that's where she got it and which product it was so I'm going to take the supplier's MSDS as the more authoritative.I am sorry but your figures are wrong.
Without a real test to figure the SAP value it's academic anyway, since we don't know the level of impurities. Real Soon Now™ I'm going to get the rest of what I need to do those titrations.
Does the average soaper need to do that? I'd argue not. It is part of what I enjoy about this though - learning the science behind the craft. I approach cooking, music, pyrotechnics, all the things I really love in the same manner. It's definitely a product of some OCD tendencies.
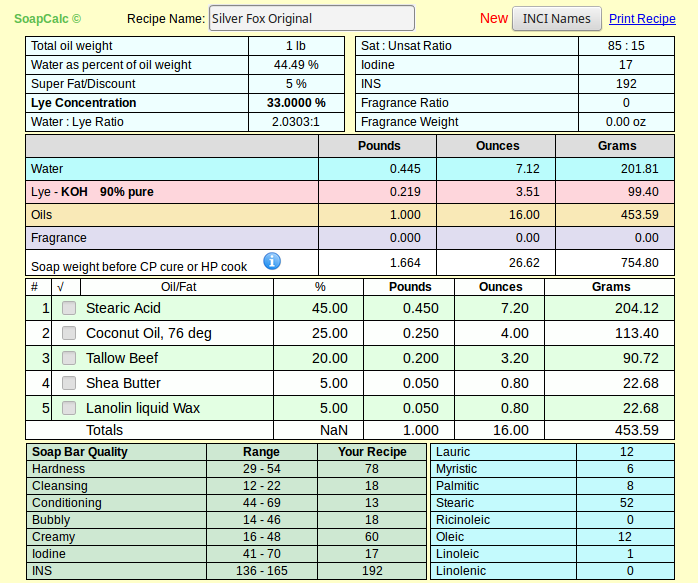
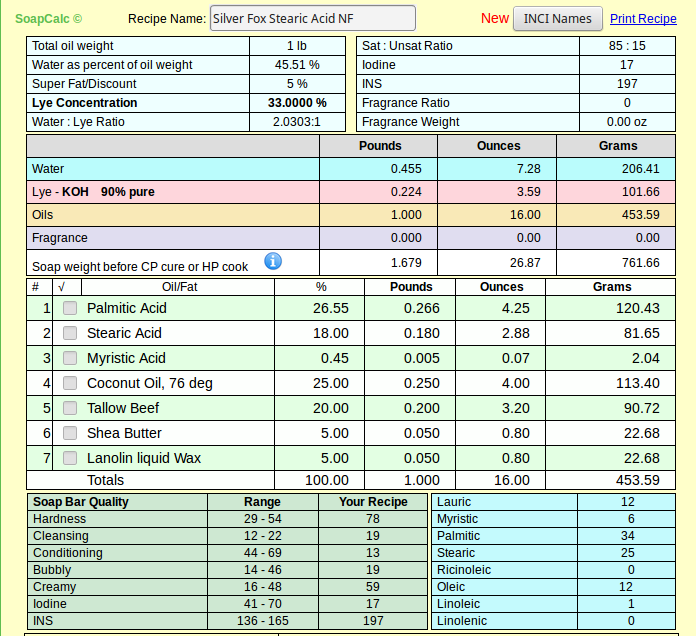
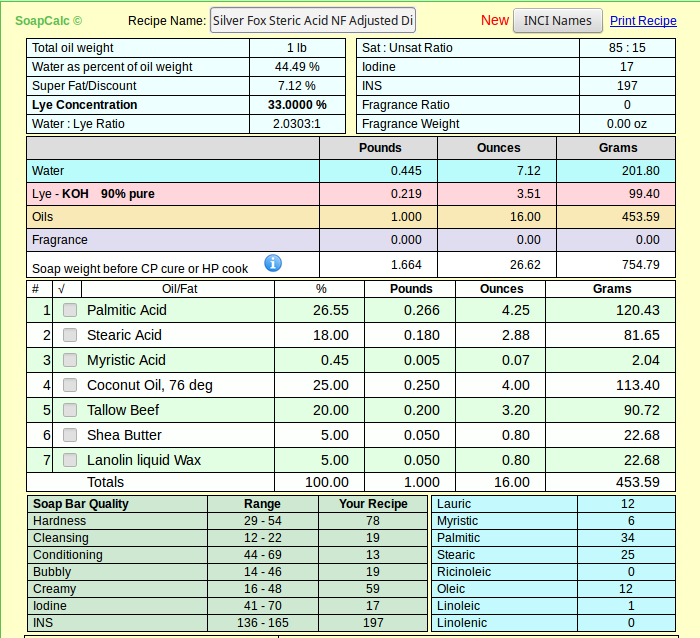
The food grade I quoted is not any more or less pure than the other - we just happen to be lucky enough to have a craft supplier that can buy in bulk and pass those savings along to us. I suspect if I want to buy around 250# I can get the same price for food grade SA.As for price what can you expect, you have to pay for purity, and its you who want to use it. Most stearic acid sold for soap making is the 60% palmitic acid / 40% stearic acid, which i have used for several years and most discussions on this form and others about shaving soaps are based.
And please don't get me wrong - I know there's nothing wrong with the product as sold and used by soapers since long before this wet-nosed soaper got here. It's 55% curiosity and 45% because I just happened to create a recipe I like with real SA. If at the end I find I can't source it in quantities for the average person, I'll revise my articles (and my recipe) accordingly.