Question for you soy wax users. I just found a bag of Aztec LP 416. I think I was thinking of diving into wax tarts at some point and it was just all too much for me. So, here this bag sits. Can this be used in soaping? I'vte looked on their website and it says "This soy wax contains no additives and is both natural and kosher. This wax blends well with beeswax, paraffin, slack wax, and microcrystalline waxes". Their 415 says, "This is a pure soy wax that contain no additives and is natural and kosher. This wax blends well with beeswax, paraffin, slack wax, and microcrystalline waxes". The only difference being the word "pure" in the 415. HELP!!! The website does not provide any additional information.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Soy Wax Users
- Thread starter KiwiMoose
- Start date

Help Support Soapmaking Forum:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
ResolvableOwl
Notorious Lyear
It's comparatively high-melting (130-135°F from what I found), i. e. very effective for hard soap, but it might be stubborn in CP recipes (false trace). Still sounds like worth a try!
I agree - just don't soap too cool - I wouldn't let it get under 100 degrees in your language. Let us know and report back! It's cheaper than 415 so I'd be interested to know.
Zany_in_CO
Saponifier
I agree with @ResolvableOwl & @KiwiMoose -- looks fine to me. Personally, I would limit its use to 10% in CP and aim for INS of 145 - 160 on SoapCalc, but that's just me.Can this be used in soaping?
Other practical uses for soy wax:
Vegan Lip Balm
Gardener's Hand Balm presented by P. Allen Smith (an actual gardener!
So I got my PureSoy S100, from an European online seller (one of Premium Soy Wax For Candles | All Seasons Wax Company distributors). It came in a plastic bag, with no label. It smelled like stearin candles. Well, I hope it is the PureSoy s100.
I melted the CO first and then 10 % SW (it melted fast). After it all melted I added the other oils. I mixed the oils all the time but I also stickblended them (anxiety much..) I waited until 37 °C (98.6 °F) before adding the lye (that was about 40 °C/104 °F) and it still was clear (not opaque). I made a very light trace before I poured. I also added 1 % table salt, 2 % citric acid.
N.B. In the recipe I used a lot of canola oil because I was afraid that it would trace too fast. Lye conc was 34 %.
I made a small batch in a silicon loaf that I covered with a plastic film. I unmolded and cut the loaf after about 16 hours. It was still a bit soft, which usually do not happen when I add salt.
Picture nr 1: the end of the load.

Picture nr 2: the cut surface. There are air bubbles and some stearic spots? I didn't cut with a wire cutter, just an ordinary soap cutter.

I thought that PureSoy s100 was ok to work with, but I probably should stay over 40 °C/104 °F.
(btw I did a smaller batch in 6 silicon molds. I soaped around 40 °C and didn't stickblend the oils before and didn't introduce any air that way - no spots.)
I melted the CO first and then 10 % SW (it melted fast). After it all melted I added the other oils. I mixed the oils all the time but I also stickblended them (anxiety much..) I waited until 37 °C (98.6 °F) before adding the lye (that was about 40 °C/104 °F) and it still was clear (not opaque). I made a very light trace before I poured. I also added 1 % table salt, 2 % citric acid.
N.B. In the recipe I used a lot of canola oil because I was afraid that it would trace too fast. Lye conc was 34 %.
I made a small batch in a silicon loaf that I covered with a plastic film. I unmolded and cut the loaf after about 16 hours. It was still a bit soft, which usually do not happen when I add salt.
Picture nr 1: the end of the load.

Picture nr 2: the cut surface. There are air bubbles and some stearic spots? I didn't cut with a wire cutter, just an ordinary soap cutter.

I thought that PureSoy s100 was ok to work with, but I probably should stay over 40 °C/104 °F.
(btw I did a smaller batch in 6 silicon molds. I soaped around 40 °C and didn't stickblend the oils before and didn't introduce any air that way - no spots.)
Last edited:
I will definitely keep you guys posted. Plan to make a test batch this week.I agree - just don't soap too cool - I wouldn't let it get under 100 degrees in your language. Let us know and report back! It's cheaper than 415 so I'd be interested to know.

$8.62
$17.99
The Natural Soap Making Book for Beginners: Do-It-Yourself Soaps Using All-Natural Herbs, Spices, and Essential Oils
Amazon.com

$69.97
$79.99
Illumive Deluxe Soap Making Kit - Large Soap Making Kit. Includes All Soap Making Supplies. DIY Soap Making Kit.
Novarbee

$14.18 ($0.44 / Ounce)
$14.97 ($0.47 / Ounce)
Golden Barrel Butter Flavored Coconut Oil (32 oz.)
JF Distributions

$26.24 ($0.33 / Ounce)
$32.00 ($0.40 / Ounce)
Primal Elements Triple Butter Soap Base (Mango, Shea, and Cocoa Butter) - Moisturizing Melt and Pour Glycerin Soap Base for Crafting and Soap Making, Vegan, Cruelty Free, Easy to Cut - 5 Pound
Amazon.com

$37.95 ($0.34 / Ounce)
COCONUT 76 Oil. Soap making supplies. 7 pound Gallon.
Traverse Bay Bath And Body

$56.99
$69.99
WALLACE & WILLIAMSON DIY Soap Making Kit with Melt & Pour Base, Cutting Box, Molds, Fragrances, Flowers Silicone Molds - for Adults & Kids Craft
Wallace & Williamson
ResolvableOwl
Notorious Lyear
I just found another use case for soap from soy wax & friends. Back then, when I titrated the SAP of my canola wax, I had to make a slightly lye-heavy soap from it. I grated up the remainders and added them into the batter of a HP soap. High hopes back then, that it would dissolve and end up in the matrix together with the other soaping oils.
Turns out this was not the case, lol. Today I eventually started using one of these bars, and noticed they're a bit “scratchy” – in fact, the ground hard soap makes up for a mild, residue-free exfoliant, when added as fine, crumbly “confetti”.
Time will show how the superior “grip” that it lends to the bar works out, and if it slows down how quickly the bar would dissolve by the sink/shower/bathtub.
Turns out this was not the case, lol. Today I eventually started using one of these bars, and noticed they're a bit “scratchy” – in fact, the ground hard soap makes up for a mild, residue-free exfoliant, when added as fine, crumbly “confetti”.
Time will show how the superior “grip” that it lends to the bar works out, and if it slows down how quickly the bar would dissolve by the sink/shower/bathtub.
Finally made some soap with my GW415 yesterday. Went with 40%OO, 25%CO, 20% soy wax, 10% castor & 5% Shea butter. Scented with 50/50 split peppermint E/O & lavender E/O. It smells sooooooooooo good! Swirl sux but didn’t pre-plan. Had one idea & then last minute changed my mind. Can’t wait to try the flower, as it’s my tester bar for 1st time recipe.I found & bought 5lbs of Golden Foods 415, 100% soy wax!!!

I’m excited to try soy wax in some!!!

Love that colour - it's so pretty! I was just wondering how evyone is getting on with their soy wax experiments. How about yours @Rsapienza?Finally made some soap with my GW415 yesterday. Went with 40%OO, 25%CO, 20% soy wax, 10% castor & 5% Shea butter. Scented with 50/50 split peppermint E/O & lavender E/O. It smells sooooooooooo good! Swirl sux but didn’t pre-plan. Had one idea & then last minute changed my mind. Can’t wait to try the flower, as it’s my tester bar for 1st time recipe.
View attachment 61777
@KiwiMoose The color is Spring from Nurture Soap given 2me as a freebie with my latest order. The color matches the smell fantastically. Like a peppermint cream mint. 
 This was a 5 oil batch. I’d love to formulate a 4oil bar using GW415 with INS (145-160) using SAP of .144 for NaOH & using lower CO than the 25% in this 1st test batch. I think I may have to wait until after Christmas however. I still have soap dishes to make.
This was a 5 oil batch. I’d love to formulate a 4oil bar using GW415 with INS (145-160) using SAP of .144 for NaOH & using lower CO than the 25% in this 1st test batch. I think I may have to wait until after Christmas however. I still have soap dishes to make.

Last edited by a moderator:
Love that colour - it's so pretty! I was just wondering how evyone is getting on with their soy wax experiments. How about yours @Rsapienza?
I finally made it! I made a small batch, 24 oz oils. I used a MB 50/50 lye solution that had sugar and silk already added to it. I used CM for the remaining liquid. I used a 36%lye concentration.
23% RBO
20% Soy Wax
16% CO
15% Mango Butter
11% SAO
10% SB
5% Castor
I had some oils and butters I need to get rid of…LOL, hence the variety
It did thicken up quite a bit. I had to really bang my mold, but other than that, things went pretty smooth. I scented with Dewberry from WSP and added right before pour. I took a small shaving and washed my hands with it. It didn’t seem to produce too many bubbles, but we’ll see what happens after cure.
Attachments
In my family ( I have three sisters) SB is used on messenger to mean 'Stupid Bi***' in a friendly, sisterly way of course  What does it mean in your recipe?
What does it mean in your recipe?
ResolvableOwl
Notorious Lyear
I wondered too. After some thorough research, I've concluded she indeed means finely ground stick blenders. (a boring second place goes to shea butter, via the infamous abbreviation list).
Last edited:
Shea ButterIn my family ( I have three sisters) SB is used on messenger to mean 'Stupid Bi***' in a friendly, sisterly way of courseWhat does it mean in your recipe?
Oh I see! Now i really do feel like a SBShea Butter
ResolvableOwl
Notorious Lyear
An unknowing parcel delivery guy has handed over this early christmas present to me:

Per the article description, it is canola oil, partially hydrogenated to an iodine value of 45–55.
Yes! Finally some numbers to work with .
.
My first impulse was to ask the manufacturer if they wouldn't have a GC analysis/FA profile to share with me. On the other hand, as long as we have only a rough conception how really the elaidic acid works (wrt soap hardness), a precise profile isn't too helpful anyway – we wouldn't gain much beyond guesstimates.
So, let's guesstimate on our own!
We can get this interesting graphic from this publication:
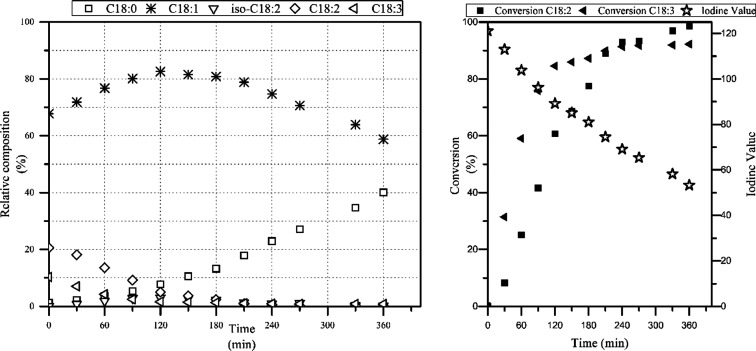
Composition change of canola oil during hydrogenation. Unfortunately C18:1 isn't further broken down to oleic/elaidic. But we see the IV go down from about 120 to 50 (stars in the right panel) – exactly where OBW073 is located! At that 360 minute mark, on the left, we see that we can expect next to zero remaining PUFAs as well as about 40% stearic acid.
Now, in contact with hydrogenation catalysts, there is an equilibrium between oleic and elaidic acid. How this looks exactly is tricky to estimate (and depends on everything: reaction conditions, catalysts and time in particular). The best I could find on the quick was this paper. They however stop too early at IV ≈ 70. But the trend they see is that elaidic levels rise about twice as fast as the stearic (which is of limited use in the IV regions we're looking at). I loosely remember that, in equilibrium, cis-trans isomerisation favours the trans variant by somewhere 2:1…5:1.
Once again, exact values aren't even particularly helpful as long as we don't know how to treat elaidic acid in terms of its properties. It is evident that it is “harder” than oleic acid, but on the other hand it's also less hardening than stearic acid.
This is, for now, my approach to this situation: I theoretically divide the C18:1 part 2:1 into 40% elaidic and 20% oleic acid. I also assume that elaidic acid is about half the way between oleic and stearic acid, i. e. 2 g elaidic acid contribute to hardness/longevity/creamy numbers like 1 g stearic acid. For this canola wax, I end up with a working hypothesis of effective 60% stearic acid content.
How convenient that there is an oil in soap calculators that matches these properties quite well! Kokum butter is listed with 56% stearic (close enough to my guesstimate), 4% palmitic (exactly as canola), and the remainder nearly pure oleic acid. IV and INS are, of course, off the chart. The SAP is 0.135, a tiny bit too high for the 0.133 of canola oil, but reasonably close to offset with some 0.5% superfat discount (at typical usage rates). The melting range of kokum butter (37–40°C) is quite a bit lower than the specification of the OBW073 (47–54°C); we'll see how this pans out, especially in oil blends. Anyway, a reminder for the annoyances of false trace can never be wrong .
.
Needless to say that I will keep you well supplied with updates how the OBW073 is performing!

Per the article description, it is canola oil, partially hydrogenated to an iodine value of 45–55.
Yes! Finally some numbers to work with
My first impulse was to ask the manufacturer if they wouldn't have a GC analysis/FA profile to share with me. On the other hand, as long as we have only a rough conception how really the elaidic acid works (wrt soap hardness), a precise profile isn't too helpful anyway – we wouldn't gain much beyond guesstimates.
So, let's guesstimate on our own!
We can get this interesting graphic from this publication:

Composition change of canola oil during hydrogenation. Unfortunately C18:1 isn't further broken down to oleic/elaidic. But we see the IV go down from about 120 to 50 (stars in the right panel) – exactly where OBW073 is located! At that 360 minute mark, on the left, we see that we can expect next to zero remaining PUFAs as well as about 40% stearic acid.
Now, in contact with hydrogenation catalysts, there is an equilibrium between oleic and elaidic acid. How this looks exactly is tricky to estimate (and depends on everything: reaction conditions, catalysts and time in particular). The best I could find on the quick was this paper. They however stop too early at IV ≈ 70. But the trend they see is that elaidic levels rise about twice as fast as the stearic (which is of limited use in the IV regions we're looking at). I loosely remember that, in equilibrium, cis-trans isomerisation favours the trans variant by somewhere 2:1…5:1.
Once again, exact values aren't even particularly helpful as long as we don't know how to treat elaidic acid in terms of its properties. It is evident that it is “harder” than oleic acid, but on the other hand it's also less hardening than stearic acid.
This is, for now, my approach to this situation: I theoretically divide the C18:1 part 2:1 into 40% elaidic and 20% oleic acid. I also assume that elaidic acid is about half the way between oleic and stearic acid, i. e. 2 g elaidic acid contribute to hardness/longevity/creamy numbers like 1 g stearic acid. For this canola wax, I end up with a working hypothesis of effective 60% stearic acid content.
How convenient that there is an oil in soap calculators that matches these properties quite well! Kokum butter is listed with 56% stearic (close enough to my guesstimate), 4% palmitic (exactly as canola), and the remainder nearly pure oleic acid. IV and INS are, of course, off the chart. The SAP is 0.135, a tiny bit too high for the 0.133 of canola oil, but reasonably close to offset with some 0.5% superfat discount (at typical usage rates). The melting range of kokum butter (37–40°C) is quite a bit lower than the specification of the OBW073 (47–54°C); we'll see how this pans out, especially in oil blends. Anyway, a reminder for the annoyances of false trace can never be wrong
Needless to say that I will keep you well supplied with updates how the OBW073 is performing!
Eeeeek! I fell asleep halfway through, but I do want to understand. So in layperson's terms - can I use it instead of soy wax and will it provide similar or different properties? Shall I do a sample recipe using Kokum butter as the 'replacement in the calculator?An unknowing parcel delivery guy has handed over this early christmas present to me:
View attachment 63165
Per the article description, it is canola oil, partially hydrogenated to an iodine value of 45–55.
Yes! Finally some numbers to work with.
My first impulse was to ask the manufacturer if they wouldn't have a GC analysis/FA profile to share with me. On the other hand, as long as we have only a rough conception how really the elaidic acid works (wrt soap hardness), a precise profile isn't too helpful anyway – we wouldn't gain much beyond guesstimates.
So, let's guesstimate on our own!
We can get this interesting graphic from this publication:

Composition change of canola oil during hydrogenation. Unfortunately C18:1 isn't further broken down to oleic/elaidic. But we see the IV go down from about 120 to 50 (stars in the right panel) – exactly where OBW073 is located! At that 360 minute mark, on the left, we see that we can expect next to zero remaining PUFAs as well as about 40% stearic acid.
Now, in contact with hydrogenation catalysts, there is an equilibrium between oleic and elaidic acid. How this looks exactly is tricky to estimate (and depends on everything: reaction conditions, catalysts and time in particular). The best I could find on the quick was this paper. They however stop too early at IV ≈ 70. But the trend they see is that elaidic levels rise about twice as fast as the stearic (which is of limited use in the IV regions we're looking at). I loosely remember that, in equilibrium, cis-trans isomerisation favours the trans variant by somewhere 2:1…5:1.
Once again, exact values aren't even particularly helpful as long as we don't know how to treat elaidic acid in terms of its properties. It is evident that it is “harder” than oleic acid, but on the other hand it's also less hardening than stearic acid.
This is, for now, my approach to this situation: I theoretically divide the C18:1 part 2:1 into 40% elaidic and 20% oleic acid. I also assume that elaidic acid is about half the way between oleic and stearic acid, i. e. 2 g elaidic acid contribute to hardness/longevity/creamy numbers like 1 g stearic acid. For this canola wax, I end up with a working hypothesis of effective 60% stearic acid content.
How convenient that there is an oil in soap calculators that matches these properties quite well! Kokum butter is listed with 56% stearic (close enough to my guesstimate), 4% palmitic (exactly as canola), and the remainder nearly pure oleic acid. IV and INS are, of course, off the chart. The SAP is 0.135, a tiny bit too high for the 0.133 of canola oil, but reasonably close to offset with some 0.5% superfat discount (at typical usage rates). The melting range of kokum butter (37–40°C) is quite a bit lower than the specification of the OBW073 (47–54°C); we'll see how this pans out, especially in oil blends. Anyway, a reminder for the annoyances of false trace can never be wrong.
Needless to say that I will keep you well supplied with updates how the OBW073 is performing!
ETA - I just did that and it affects my hardness and longevity numbers ( although we know that soy wax is not accurately represented in the calculators so it may actually be similar) - it reduces it by about 7% and also the creamy lather by about the same. However, the conditioning increases by about 10%. Your thoughts?
Last edited:
ResolvableOwl
Notorious Lyear
Essentially the same reasoning should apply to soy wax. I have no access to actual soy wax (honestly, I don't care either) to compare partially hydrogenated soy vs. canola, but for now I assume they can be treated mostly the same. AFAICS the GW415 is close enough to OBW073 (final IV, melting range) – that also makes sense from its on-label use as candle fuel!
Be assured that swapping the name of soy wax from “fully hydrogenated soy wax” to “kokum butter” will not change your soap! It does just change the numbers that the calculator spits out, hence possibly the way in which you are interpreting these numbers in relation to the observations of your physical soaps (for sure you yourself know your soaps better than the makers of the calculators ). Unless you're unhappy with your soaps, there is no need to actually modify your recipes.
). Unless you're unhappy with your soaps, there is no need to actually modify your recipes.
My kokum trick is based on … well, laziness. In my observations, the fully hydrogenated option isn't a good choice for “soft” vegetable-oil-waxes, since they provide significantly less hardening than, say, fully hydrogenated oils or FFA-type palm stearin (technical palmitic acid). Until recently, I usually eyeballed it and divided the portions of my (retail-candle harvested) canola wax by a mix of 100% and 27.5% hydrogenated soy. Now that I got through all the numbers, I'm now somewhat confident that kokum butter is an easier yet about as accurate replacement. Time to challenge my recent optimism with reality .
.
tl;dr:
IV≈50: soft oil waxes (soy, canola … based) ≈ use “kokum butter”
IV≈0: hard oil waxes ≈ use “Soybean, fully hydrogenated (soy wax)”
unless there is evidence to do better.
Be assured that swapping the name of soy wax from “fully hydrogenated soy wax” to “kokum butter” will not change your soap! It does just change the numbers that the calculator spits out, hence possibly the way in which you are interpreting these numbers in relation to the observations of your physical soaps (for sure you yourself know your soaps better than the makers of the calculators
My kokum trick is based on … well, laziness. In my observations, the fully hydrogenated option isn't a good choice for “soft” vegetable-oil-waxes, since they provide significantly less hardening than, say, fully hydrogenated oils or FFA-type palm stearin (technical palmitic acid). Until recently, I usually eyeballed it and divided the portions of my (retail-candle harvested) canola wax by a mix of 100% and 27.5% hydrogenated soy. Now that I got through all the numbers, I'm now somewhat confident that kokum butter is an easier yet about as accurate replacement. Time to challenge my recent optimism with reality
tl;dr:
IV≈50: soft oil waxes (soy, canola … based) ≈ use “kokum butter”
IV≈0: hard oil waxes ≈ use “Soybean, fully hydrogenated (soy wax)”
unless there is evidence to do better.
ResolvableOwl
Notorious Lyear
Some details to the soy wax part of my scented candle thread:
With a hugely scientific sample size of 1 (plus, unknown wax type, and unknown FOs/additives), my experience was that (this) soy is harder/stiffer/less “sticky” when cold/solid with a more even and very appealing gloss (that whoever made candle managed to completely ruin with an obviously inappropriate cooling protocol).
The soy melts slower, and once molten/in a soap batter, it was slightly more prone to solidification/false trace than the canola waxes I've worked with so far.
I'm also under the impression that, once in the soap (settling/unmoulding/early curing), it is slower to “bite” wrt hardness/solidification of the young soap. I unmoulded the soap columns after CPOP + 4 days of waiting (distracted by the holidays ). Even then, they were just barely hard enough to not entirely lose their shape.
). Even then, they were just barely hard enough to not entirely lose their shape.
All the ugly ends and top pieces got squeezed into that hexagonal bar (I should probably post a tutorial how to do this, once I'm confident enough how to shoot it without contaminating my camera, lol).
Bottom line: This soy wax might behave ideal for candles (I haven't tested it in a candle, but canola wax isn't always the most cooperative here). When it comes to soap, canola wax appears to be similar, or slightly more easily to work with in pretty much all respects.
Sounds like I'll stay with canola wax for the time being.
With a hugely scientific sample size of 1 (plus, unknown wax type, and unknown FOs/additives), my experience was that (this) soy is harder/stiffer/less “sticky” when cold/solid with a more even and very appealing gloss (that whoever made candle managed to completely ruin with an obviously inappropriate cooling protocol).
The soy melts slower, and once molten/in a soap batter, it was slightly more prone to solidification/false trace than the canola waxes I've worked with so far.
I'm also under the impression that, once in the soap (settling/unmoulding/early curing), it is slower to “bite” wrt hardness/solidification of the young soap. I unmoulded the soap columns after CPOP + 4 days of waiting (distracted by the holidays
All the ugly ends and top pieces got squeezed into that hexagonal bar (I should probably post a tutorial how to do this, once I'm confident enough how to shoot it without contaminating my camera, lol).
Bottom line: This soy wax might behave ideal for candles (I haven't tested it in a candle, but canola wax isn't always the most cooperative here). When it comes to soap, canola wax appears to be similar, or slightly more easily to work with in pretty much all respects.
Sounds like I'll stay with canola wax for the time being.
I noticed tonight that Candle Science is carrying Ecosoya wax. They’re getting it from a company called Kerax in the U.K. Does anyone know this company‘s wax? The pillar blend is described as 100% soy wax and it melts at 131F, so a little bit higher than 415, which melts at 121-125F. If there are no additives, as described, and the melting point is higher, is the %stearic likely to be higher than it is in 415? If so, I might give it a try.
Similar threads
- Replies
- 22
- Views
- 580




































