snappyllama
Supporting Member
Hi folks,
Last night was my first time making Pine Tar soap, and I had a hiccup along the way. Now I have PT soap that looks lighter and more speckled than the other ones I've seen here.
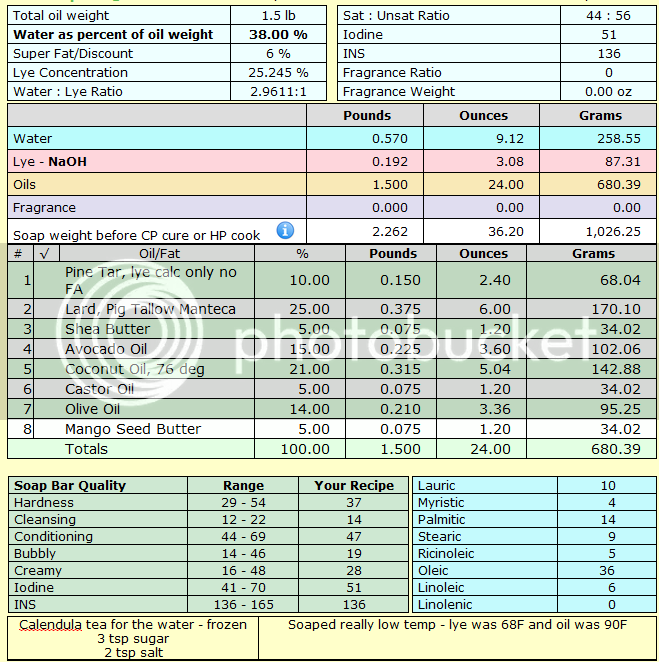
I've used the recipe once before with good results. I made 1.5lb batch.
All water was subbed out for calendula tea that I made by soaking dried petals in hot distilled water, straining, and then freezing. I added the lye to semi-melted calendula ice cubes and stirred until both are completely dissolved. Then I added 3 tsp sugar and 2 tsp salt and stirred until those are completely dissolved.
Last time, my lye water was at a perfect 90F when done with this step. This time, I was interrupted for several hours and my lye water was around 65F when I got back. I gave it a good stir, and all seemed well, but I did strain out what looked like a thin, crystal flake. I'm guessing/hoping it was some sugar. My oils were at 90. I've read here to not worry too much about temperature differences so I trod on, confident and sleepy.
I added my pine tar at medium trace (it took forever to just to get to trace), hand stirred and seemed to have plenty of time to play so, I hit it with a stick blender too.
I've tested it out, no zap, light smokey scent (I actually rather like it), small/ creamy bubbles.
So for the novella... my question is: do you think the tar attached itself to the calendula bits in the water and that's the speckles? I colored my previous calendula water batch so I don't know if I had speckles there too...
Recipe:
Soaps:


Last night was my first time making Pine Tar soap, and I had a hiccup along the way. Now I have PT soap that looks lighter and more speckled than the other ones I've seen here.
I've used the recipe once before with good results. I made 1.5lb batch.
All water was subbed out for calendula tea that I made by soaking dried petals in hot distilled water, straining, and then freezing. I added the lye to semi-melted calendula ice cubes and stirred until both are completely dissolved. Then I added 3 tsp sugar and 2 tsp salt and stirred until those are completely dissolved.
Last time, my lye water was at a perfect 90F when done with this step. This time, I was interrupted for several hours and my lye water was around 65F when I got back. I gave it a good stir, and all seemed well, but I did strain out what looked like a thin, crystal flake. I'm guessing/hoping it was some sugar. My oils were at 90. I've read here to not worry too much about temperature differences so I trod on, confident and sleepy.
I added my pine tar at medium trace (it took forever to just to get to trace), hand stirred and seemed to have plenty of time to play so, I hit it with a stick blender too.
I've tested it out, no zap, light smokey scent (I actually rather like it), small/ creamy bubbles.
So for the novella... my question is: do you think the tar attached itself to the calendula bits in the water and that's the speckles? I colored my previous calendula water batch so I don't know if I had speckles there too...
Recipe:

Soaps:

















































