xoticsoaps
Well-Known Member
- Joined
- May 23, 2014
- Messages
- 92
- Reaction score
- 23
Using the chart below, what would be the sap value of Bacuri butter if you're using sodium hydroxide?
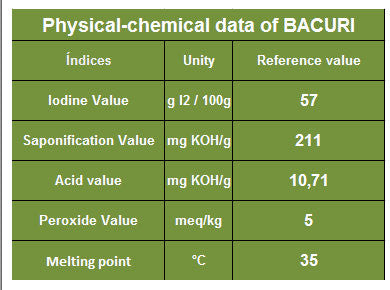



Using the chart below, what would be the sap value of Bacuri butter if you're using sodium hydroxide?

@DeeAnna - Thank you for the help.
@Dorymae - I got the same.
211/1.403 = 150.39
If I were to input it into a lye calculator would it be 150.3 or 150.39??
where does the 1.403 come from?Divide the KOH sap value by 1.403.














So 1.403 is a fixed number?To saponify any fat, you will need to count out "X" molecules of KOH or the same "X" molecules of NaOH. If you could do that -- actually count the molecules -- and weigh them, you will find the KOH molecules will weigh 1.403 more than the same number of NaOH molecules.
A chemist would say it this way -- 1.403 is the ratio of the molecular weight of KOH divided by the molecular weight of NaOH.
Enter your email address to join: