Hi All. I made some lard soap the other day. Every one else I spoke with said my bar should be hard. I just un-molded it yesterday and it feels "spongy". I have made vegan soap before and that is usually harder. Does lard soap take longer to cure? Does it have a different feel to the finished product than a vegan soap? :-?
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
New to using lard
- Thread starter DanaT
- Start date

Help Support Soapmaking Forum:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
Britannic
Ancient Briton
Post your full recipe, including water and sodium hydroxide weights. Hard to tell without the recipe why yours is spongy. Did you hot process or cold process the soap, did a lot of air get whipped into the batter?
My lard based recipes are firm and cuttable within 12-14 hours, but usually become rock hard as they age.
My lard based recipes are firm and cuttable within 12-14 hours, but usually become rock hard as they age.
Rusti
Well-Known Member
Most CP soap is firm enough to cut in a few hours to a day, but feels 'spongy' for at least a couple of weeks if not longer while it cures. I've never had a bar of soap that I thought felt 'hard' until after it was done curing.
Hi Brittanic. Here's the recipe. It's Soleseife (brine) soap:
10.25 oz lard
12.3 oz coconut oil
12.3 oz olive oil
4.1 oz safflower oil
2.05 oz castor oil
14.5 oz water
5.8 oz lye (5% discount) - whatever that means
3.6 oz plain sea salt
1.8 oz f/o - I added lavender E/O
Even with salt added it should have made the bar hard
10.25 oz lard
12.3 oz coconut oil
12.3 oz olive oil
4.1 oz safflower oil
2.05 oz castor oil
14.5 oz water
5.8 oz lye (5% discount) - whatever that means
3.6 oz plain sea salt
1.8 oz f/o - I added lavender E/O
Even with salt added it should have made the bar hard
BrewerGeorge
Well-Known Member
No offense, but it's going to to take me a bit to convert that into something I can read (ie grams) before I can comment. :twisted:Hi Brittanic. Here's the recipe. It's Soleseife (brine) soap:
10.25 oz lard
12.3 oz coconut oil
12.3 oz olive oil
4.1 oz safflower oil
2.05 oz castor oil
14.5 oz water
5.8 oz lye (5% discount) - whatever that means
3.6 oz plain sea salt
1.8 oz f/o - I added lavender E/O
Even with salt added it should have made the bar hard
Britannic
Ancient Briton
Hi Brittanic. Here's the recipe. It's Soleseife (brine) soap:
10.25 oz lard
12.3 oz coconut oil
12.3 oz olive oil
4.1 oz safflower oil
2.05 oz castor oil
14.5 oz water
5.8 oz lye (5% discount) - whatever that means
3.6 oz plain sea salt
1.8 oz f/o - I added lavender E/O
Even with salt added it should have made the bar hard
Hot or cold process? The lard percentage (25%) is less than than the olive oil (30%) and coconut (30%). Olive Oil starts out soft before it hardens. Spongy soap can be caused by heavy stick blending and accidentally incorporating too much air if the batter is already thickening (been there and done that). Can you tell us more about how you processed your soap?
It may be spongy now, but based on my own experience, even my super aerated batch ended up rock hard after 6 weeks.

$13.59 ($0.42 / Fl Oz)
$15.99 ($0.50 / Fl Oz)
Viva Naturals Organic Coconut Oil - Unrefined and Cold-Pressed, Natural Hair Oil, Skin Oil and Cooking Oil with Fresh Flavor, Non-GMO Extra Virgin Coconut Oil (Aceite de Coco), USDA Organic, 32 Fl Oz (Pack of 1)
Amazon.com

$25.99 ($0.32 / Ounce)
velona 5 LB - Shea Butter - Melt and Pour Soap Base SLS/SLES free | Natural Bars for The Best Result for Soap-Making
Velona

$7.99 ($2.00 / Fl Oz)
$9.99 ($2.50 / Fl Oz)
SilkySecret Peppermint Essential Oil (4 Fl Oz), Mint Oil for Hair Care, Skin Massage, Aromatherapy and Sprays, Relieves Muscle Pain, Refreshes
Saahow

$18.99 ($37.98 / Fl Oz)
US Organic 100% Pure Lavender Essential Oil, Directly sourced from Bulgaria, USDA Certified Organic, Undiluted, for Diffuser, Humidifier, Massage, Skin, Hair Care, Non GMO, 15 ml
US Organic Group Corp

$35.74 ($0.32 / Ounce)
Nature's Oil Coconut 76 Degree, Naturally Refined, 7lbs (1 Gallon)
Bulk Apothecary

$37.95 ($0.34 / Ounce)
COCONUT 76 Oil. Soap making supplies. 7 pound Gallon.
Traverse Bay Bath And Body

$25.60 ($0.32 / Ounce)
$32.00 ($0.40 / Ounce)
Primal Elements Triple Butter Soap Base (Mango, Shea, and Cocoa Butter) - Moisturizing Melt and Pour Glycerin Soap Base for Crafting and Soap Making, Vegan, Cruelty Free, Easy to Cut - 5 Pound
Amazon.com

$11.99 ($3.00 / Fl Oz)
Ethereal Nature 100% Pure! Peppermint Oil – Perfect For Aromatherapy Diffusers, Skin, Nail and Hair Care – Beauty DIY – 4 FL OZ
Amazon.com

$8.62
$14.99
The Natural Soap Making Book for Beginners: Do-It-Yourself Soaps Using All-Natural Herbs, Spices, and Essential Oils
Amazon.com

$7.99
$9.99
KT THERMO Instant Read 1-Inch Dial Thermometer,Best for The Coffee Drinks,Chocolate Milk Foam
BestPartners

$6.74 ($0.48 / Fl Oz)
La Tourangelle, Organic Coconut Oil, Refined, For Cooking, Baking, Hair, and Skin Care, Expeller Pressed, 14 fl oz
Amazon.com

$13.59 ($0.47 / Fl Oz)
$16.99 ($0.59 / Fl Oz)
Nutiva Organic Coconut Oil with Non-Dairy Butter Flavor, 29 Fl. Oz. USDA Organic, Non-GMO, Whole 30 Approved, Vegan & Gluten-Free, Plant-Based Replacement for Butter
Amazon.com

$16.95 ($4.24 / Fl Oz)
Pure Body Naturals French Lavender Essential Oil Blend, 4 fl oz - for Aromatherapy, Soap Making, and DIY Skin and Hair Products
Pure Body Naturals®
Britannic
Ancient Briton
I plugged your recipe into Soapee.com to help others with the percentages/grams etc.


Last edited:
BrewerGeorge
Well-Known Member
...
ETA: Aw, Man! I left out the castor. Deleting the rest because Britannic
ETA: Aw, Man! I left out the castor. Deleting the rest because Britannic
BattleGnome
Well-Known Member
- Joined
- Jan 3, 2016
- Messages
- 1,666
- Reaction score
- 1,513
It can take me 5 days to be able to unmold some soaps. The I usually leave it on the counter for another day before I cut it. Sometimes all you need is time.
styarr
Active Member
- Joined
- Apr 7, 2017
- Messages
- 29
- Reaction score
- 35
It can take me 5 days to be able to unmold some soaps. The I usually leave it on the counter for another day before I cut it. Sometimes all you need is time.
second this! some soap just takes longer, I usually leave my lard soaps for 2 days before unmolding (I do use a higher % of lard but you used more water then I normally do)
It's also not a small amount of water. I know that the salt also has to have "space" in the water to dissolve, but that is a bit more than I would use. Especially, as others have said, that the lard amount is so small. A lard soap for me is about 50% lard.
Britannic
Ancient Briton
cherrycoke216
Well-Known Member
- Joined
- Jun 22, 2014
- Messages
- 561
- Reaction score
- 461
YMMV, but I have used this guide for determining lye concentrations for my cold process recipes. Obviously, some adjustment would be needed for adding salts in high concentrations.
Is it just me or the japudo.com.br won't work? I have tried multiple times, and it's always shows error connection timed out message.
My guide has always just been "what am I making and what do I want to do?"
Time to swirl, more water.
No gel, less water.
More soft oils, less water.
Shorter time in the mould, less water.
Pine tar, it doesn't matter anyway, just pray!
When you want to swirl and gel a recipe with lots of soft oils, you have to find the balance!
Time to swirl, more water.
No gel, less water.
More soft oils, less water.
Shorter time in the mould, less water.
Pine tar, it doesn't matter anyway, just pray!
When you want to swirl and gel a recipe with lots of soft oils, you have to find the balance!
Dahila
Well-Known Member
I do not gell so the last soap took 60 hours before I could cut it in logs. In a week it is going to be hard as a rock. Good soap has never too much Coconut oil (it makes it hard) and your soap is going to be hard but high cleansing too. 30% of CO.
Next one go lower on CO
Next one go lower on CO
Is it just me or the japudo.com.br won't work? I have tried multiple times, and it's always shows error connection timed out message.
Sad to say, you're right. It looks like the website was abandoned in late 2016 or early 2017. The Wayback Machine has archived copies of this website, but images aren't showing up for some reason. Here's a link to the archived page, for what it's worth: https://web.archive.org/web/2016022...13/05/14/the-importance-of-lye-concentration/
I saved a personal copy of Roberto's article "The Importance of Lye Concentration" that contains the table of lye concentration vs other soaping variables. See the attached PDF.
View attachment Akira Robt Importance of lye concentration.pdf
Is it just me or the japudo.com.br won't work? I have tried multiple times, and it's always shows error connection timed out message.
Sad to say, you're right. It looks like the website was abandoned in late 2016 or early 2017. The Wayback Machine has archived copies of this website, but images aren't showing up for some reason. Here's a link to the archived page, for what it's worth: https://web.archive.org/web/2016022...13/05/14/the-importance-of-lye-concentration/
I saved a personal copy of Roberto's article "The Importance of Lye Concentration" that contains the table of lye concentration vs other soaping variables. See the attached PDF.
I have no trouble opening the link. I also already had it bookmarked, too, so I'm glad it's still working for me.
http://www.japudo.com.br/en/2013/05/14/the-importance-of-lye-concentration/
But to be on the safe side, I've also saved the chart itself as a jpeg to my computer for future reference.
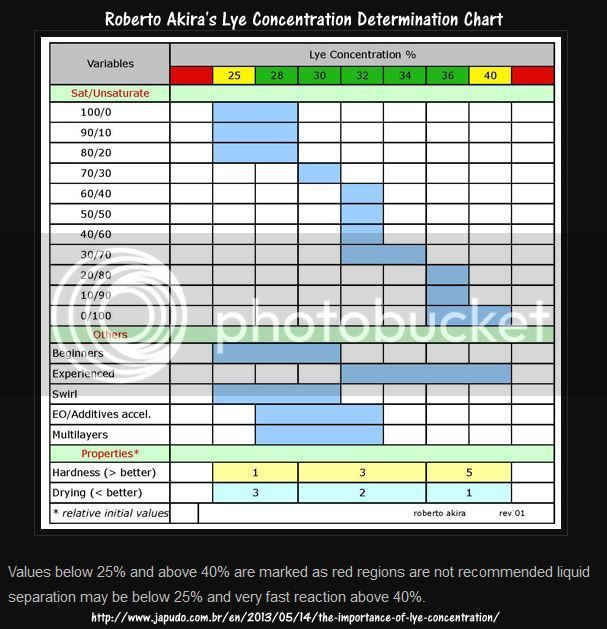
DeeAnna, thank you to your link to the PDF file. I have saved it also for future reference in case the japudo.com.br site becomes unavailable to me. I'm not sure what's going on; maybe some sort of glitch.
Huh. It's working for me now too, even without refreshing my browser's cache.
When I checked earlier today, I tried the base URL as well as the URL for the article in Britannic's link and got errors no matter what page I tried to view. I was also seeing similar errors for some of the Wayback Machine archived pages.
Go figure.
When I checked earlier today, I tried the base URL as well as the URL for the article in Britannic's link and got errors no matter what page I tried to view. I was also seeing similar errors for some of the Wayback Machine archived pages.
Go figure.
cherrycoke216
Well-Known Member
- Joined
- Jun 22, 2014
- Messages
- 561
- Reaction score
- 461
Thanks a bunch, DeeAnna and Earlene! I have save the file you two put up. 
Similar threads
- Replies
- 11
- Views
- 927

































